FIPEX sensors
First time resolved measurements of atomic and molecular oxygen were taken by the Flux-(Φ-Phi)-Probe-Experiments (FIPEX). It operated 572 days on European Technology Exposure Facility (EUTEF) on the International Space Station (ISS), fulfilled its primary objectives and collected complete reasonable data.
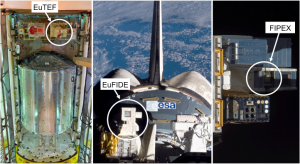
These AO and O2 sensors and in addition the new ozone sensor will operate in our experiment in a miniaturized form.
They are solid electrolyte sensors based on amperometric combined with the potentiometric-Nernst-principle for polarization control.
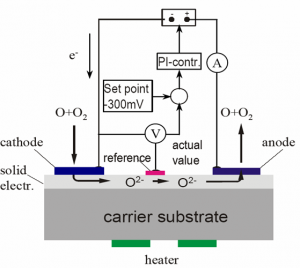
Simplified cathode reaction:
In case of contact of the gaugeable species with the cathode the impressed voltage between cathode and anode leads to a current. This is because the molecule or atom take electrons from the cathode and transport them to the anode.
The current can be measured and compared with diagrams, which were created for different partial pressures of the gaugeable species and different static pressures of the gas composition.
Difference between the oxygen sensors
The difference between the atomic and molecular oxygen sensor are the electrodes.
Atomic oxygen prefers reaction with gold electrodes however atomic and molecular oxygen prefers reaction with platinum electrodes.
So cermet electrodes on base of gold or platinum can allow a distinction between atomic and molecular oxygen.
The feedback control of the reduced potential with a reference electrode is very important because of the higher electrode polarisation in the gold electrode. The Sensor for O2 and O3 does not need this reference.
Ozone sensor
The ozone sensor works on another principle. But we must not tell more about it, because it is in a patent process.
Summary
The development of these precise sensors is an important step for better understanding of the complex and dynamic character of our atmosphere.
By means of this you can make precise prediction of specific gas densities, for example corrosive atomic oxygen.
That leads to a better assessment of necessary safeguards for long-term missions in the low earth orbit.
In addition we can better understand climatie effects which will lead to a better prediction of climatic changes and the weather.
Because of high temperatures and ultra-high vacuum in space applications, special material requisitions are given for the electrolytic conductors concerning for example electric and ionic conductibility, catalytic activity, resistance to temperature change and chemical stability of oxidative and reductive processes. Gas exchange must be possible even at low concentrations.
Measurement principle of the oxygen sensor
Oxygen molecules are adsorbed on the metal electrode and diffuse to the phase interface. On this phase interface, the oxygen ions are accumulated and transferred to the electrolyte (charge transfer). Then ions diffuse along the diffusion path in the direction to the anode.
